Ozone: Difference between revisions
From Glossary of Meteorology
imported>Perlwikibot (Created page with " {{TermHeader}} {{TermSearch}} <div class="termentry"> <div class="term"> == ozone == </div> <div class="definition"><div class="short_definition">A nearly colorless ga...") |
imported>Perlwikibot No edit summary |
||
| Line 9: | Line 9: | ||
</div> | </div> | ||
<div class="definition"><div class="short_definition">A nearly colorless gas, formula O<sub>3</sub>, [[molecular weight]] 48, that appears blue in the condensed [[phase]] or at high concentration, with a characteristic odor like that of weak chlorine.</div><br/> <div class="paragraph">It is formed in the reaction between atomic [[oxygen]] and molecular oxygen: <div class="inline-formula">[[File:ams2001glos-Oe11.gif|link=|ams2001glos-Oe11]]</div>. It is a very strong absorber of [[ultraviolet radiation]], and the presence of the [[ozone layer]] in the [[upper atmosphere]] provides an [[ozone shield]] that prevents dangerous [[radiation]] from reaching the earth's surface and allows the existence of life in its present forms. Ozone, produced by photochemical reactions, is found at all altitudes in the [[atmosphere]]. The total amount of ozone in the atmosphere would correspond to less than 1 part per million if uniformly distributed, or a column amount of about 3 mm if compressed to [[sea level pressure]]. In the [[troposphere]], it is regarded as a [[pollutant]], and its presence in high concentrations can lead to respiratory stress and crop damage. Ozone is an important component of [[photochemical smog]] and can also be formed locally by the action of electrical discharges on the air. Ozone in the free troposphere often results from downward [[transport]] from the [[stratosphere]]. In the stratosphere, ozone is formed following the [[absorption]] of radiation by molecular oxygen. Its [[mixing ratio]] there can reach several parts per million, and the [[temperature inversion]] characteristic of the stratosphere is due to the strong absorption of [[energy]] by ozone molecules in this region. In the stratosphere, ozone is destroyed predominantly by [[catalytic cycles]] involving free radicals, many of which are formed as products of human activity. Ozone has several radiation absorption bands that are atmospherically important: the very intense Hartley [[band]], between 200 and 300 nm, which is responsible for much of the heating of the upper atmosphere; the Huggins bands, between 320 and 360 nm; the [[Chappuis bands]], between 450 and 650 nm; and [[infrared]] bands, centered at 4.7, 9.6, and 14.1 μm. All the above bands have been used for the [[detection]] of ozone using various [[remote sensing]] techniques. Absorption by ozone in the infrared is responsible for its effectiveness as a [[greenhouse gas]]. <br/>''See'' [[Dobson unit]].</div><br/> </div><div class="reference">Finlayson–Pitts, B. J., and J. N. Pitts 1986. Atmospheric Chemistry. Wiley–Interscience, New York, . 1098 pp. </div><br/> <div class="reference">Seinfeld, J. H., and S. N. Pandis 1998. Atmospheric Chemistry and Physics. Wiley–Interscience, New York, . 1326 pp. </div><br/> <div class="reference">Volz, A., and D. Kley 1988. Evaluation of the Montsouris series of ozone measurements made in the nineteenth century. Nature. 332. 240–242. </div><br/> | <div class="definition"><div class="short_definition">A nearly colorless gas, formula O<sub>3</sub>, [[molecular weight]] 48, that appears blue in the condensed [[phase]] or at high concentration, with a characteristic odor like that of weak chlorine.</div><br/> <div class="paragraph">It is formed in the reaction between atomic [[oxygen]] and molecular oxygen: <div class="inline-formula">[[File:ams2001glos-Oe11.gif|link=|ams2001glos-Oe11]]</div>. It is a very strong absorber of [[ultraviolet radiation]], and the presence of the [[ozone layer]] in the [[upper atmosphere]] provides an [[ozone shield]] that prevents dangerous [[radiation]] from reaching the earth's surface and allows the existence of life in its present forms. Ozone, produced by photochemical reactions, is found at all altitudes in the [[atmosphere]]. The total amount of ozone in the atmosphere would correspond to less than 1 part per million if uniformly distributed, or a column amount of about 3 mm if compressed to [[sea level pressure]]. In the [[troposphere]], it is regarded as a [[pollutant]], and its presence in high concentrations can lead to respiratory stress and crop damage. Ozone is an important component of [[photochemical smog]] and can also be formed locally by the action of electrical discharges on the air. Ozone in the free troposphere often results from downward [[transport]] from the [[stratosphere]]. In the stratosphere, ozone is formed following the [[absorption]] of radiation by molecular oxygen. Its [[mixing ratio]] there can reach several parts per million, and the [[temperature inversion]] characteristic of the stratosphere is due to the strong absorption of [[energy]] by ozone molecules in this region. In the stratosphere, ozone is destroyed predominantly by [[catalytic cycles]] involving free radicals, many of which are formed as products of human activity. Ozone has several radiation absorption bands that are atmospherically important: the very intense Hartley [[band]], between 200 and 300 nm, which is responsible for much of the heating of the upper atmosphere; the Huggins bands, between 320 and 360 nm; the [[Chappuis bands|Chappuis bands]], between 450 and 650 nm; and [[infrared]] bands, centered at 4.7, 9.6, and 14.1 μm. All the above bands have been used for the [[detection]] of ozone using various [[remote sensing]] techniques. Absorption by ozone in the infrared is responsible for its effectiveness as a [[greenhouse gases|greenhouse gas]]. <br/>''See'' [[Dobson unit]].</div><br/> </div><div class="reference">Finlayson–Pitts, B. J., and J. N. Pitts 1986. Atmospheric Chemistry. Wiley–Interscience, New York, . 1098 pp. </div><br/> <div class="reference">Seinfeld, J. H., and S. N. Pandis 1998. Atmospheric Chemistry and Physics. Wiley–Interscience, New York, . 1326 pp. </div><br/> <div class="reference">Volz, A., and D. Kley 1988. Evaluation of the Montsouris series of ozone measurements made in the nineteenth century. Nature. 332. 240–242. </div><br/> | ||
</div> | </div> | ||
Latest revision as of 16:33, 25 April 2012
ozone[edit | edit source]
A nearly colorless gas, formula O3, molecular weight 48, that appears blue in the condensed phase or at high concentration, with a characteristic odor like that of weak chlorine.
It is formed in the reaction between atomic oxygen and molecular oxygen: 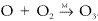 . It is a very strong absorber of ultraviolet radiation, and the presence of the ozone layer in the upper atmosphere provides an ozone shield that prevents dangerous radiation from reaching the earth's surface and allows the existence of life in its present forms. Ozone, produced by photochemical reactions, is found at all altitudes in the atmosphere. The total amount of ozone in the atmosphere would correspond to less than 1 part per million if uniformly distributed, or a column amount of about 3 mm if compressed to sea level pressure. In the troposphere, it is regarded as a pollutant, and its presence in high concentrations can lead to respiratory stress and crop damage. Ozone is an important component of photochemical smog and can also be formed locally by the action of electrical discharges on the air. Ozone in the free troposphere often results from downward transport from the stratosphere. In the stratosphere, ozone is formed following the absorption of radiation by molecular oxygen. Its mixing ratio there can reach several parts per million, and the temperature inversion characteristic of the stratosphere is due to the strong absorption of energy by ozone molecules in this region. In the stratosphere, ozone is destroyed predominantly by catalytic cycles involving free radicals, many of which are formed as products of human activity. Ozone has several radiation absorption bands that are atmospherically important: the very intense Hartley band, between 200 and 300 nm, which is responsible for much of the heating of the upper atmosphere; the Huggins bands, between 320 and 360 nm; the Chappuis bands, between 450 and 650 nm; and infrared bands, centered at 4.7, 9.6, and 14.1 μm. All the above bands have been used for the detection of ozone using various remote sensing techniques. Absorption by ozone in the infrared is responsible for its effectiveness as a greenhouse gas.
. It is a very strong absorber of ultraviolet radiation, and the presence of the ozone layer in the upper atmosphere provides an ozone shield that prevents dangerous radiation from reaching the earth's surface and allows the existence of life in its present forms. Ozone, produced by photochemical reactions, is found at all altitudes in the atmosphere. The total amount of ozone in the atmosphere would correspond to less than 1 part per million if uniformly distributed, or a column amount of about 3 mm if compressed to sea level pressure. In the troposphere, it is regarded as a pollutant, and its presence in high concentrations can lead to respiratory stress and crop damage. Ozone is an important component of photochemical smog and can also be formed locally by the action of electrical discharges on the air. Ozone in the free troposphere often results from downward transport from the stratosphere. In the stratosphere, ozone is formed following the absorption of radiation by molecular oxygen. Its mixing ratio there can reach several parts per million, and the temperature inversion characteristic of the stratosphere is due to the strong absorption of energy by ozone molecules in this region. In the stratosphere, ozone is destroyed predominantly by catalytic cycles involving free radicals, many of which are formed as products of human activity. Ozone has several radiation absorption bands that are atmospherically important: the very intense Hartley band, between 200 and 300 nm, which is responsible for much of the heating of the upper atmosphere; the Huggins bands, between 320 and 360 nm; the Chappuis bands, between 450 and 650 nm; and infrared bands, centered at 4.7, 9.6, and 14.1 μm. All the above bands have been used for the detection of ozone using various remote sensing techniques. Absorption by ozone in the infrared is responsible for its effectiveness as a greenhouse gas.
See Dobson unit.
See Dobson unit.
Finlayson–Pitts, B. J., and J. N. Pitts 1986. Atmospheric Chemistry. Wiley–Interscience, New York, . 1098 pp.
Seinfeld, J. H., and S. N. Pandis 1998. Atmospheric Chemistry and Physics. Wiley–Interscience, New York, . 1326 pp.
Volz, A., and D. Kley 1988. Evaluation of the Montsouris series of ozone measurements made in the nineteenth century. Nature. 332. 240–242.
