Potential temperature: Difference between revisions
From Glossary of Meteorology
imported>Perlwikibot (Created page with " {{TermHeader}} {{TermSearch}} <div class="termentry"> <div class="term"> == potential temperature == </div> #<div class="definition"><div class="short_definition">The ...") |
imported>Perlwikibot No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 9: | Line 9: | ||
</div> | </div> | ||
#<div class="definition"><div class="short_definition">The [[temperature]] that an unsaturated [[parcel]] of [[dry air]] would have if brought [[adiabatically]] and reversibly from its initial state to a [[standard pressure]], ''p''<sub>0</sub>, typically 100 kPa.</div><br/> <div class="paragraph">Its mathematical expression is <div class="display-formula"><blockquote>[[File:ams2001glos-Pe37.gif|link=|center|ams2001glos-Pe37]]</blockquote></div> where θ is the potential temperature, ''T'' is temperature, and κ is the [[Poisson constant]]. This exponent is often assumed to be 2/7, the ratio of the [[gas constant]] to the [[specific heat capacity]] at constant pressure for an ideal diatomic gas. <br/>''See'' [[virtual potential temperature]], [[liquid water potential temperature]], [[equivalent potential temperature]], [[wet-bulb potential temperature]].</div><br/> </div> | #<div class="definition"><div class="short_definition">The [[temperature]] that an unsaturated [[parcel]] of [[dry air]] would have if brought [[adiabatic process|adiabatically]] and reversibly from its initial state to a [[standard pressure]], ''p''<sub>0</sub>, typically 100 kPa.</div><br/> <div class="paragraph">Its mathematical expression is <div class="display-formula"><blockquote>[[File:ams2001glos-Pe37.gif|link=|center|ams2001glos-Pe37]]</blockquote></div> where θ is the potential temperature, ''T'' is temperature, and κ is the [[Poisson constant]]. This exponent is often assumed to be 2/7, the ratio of the [[gas constant]] to the [[specific heat capacity]] at constant pressure for an ideal diatomic gas. <br/>''See'' [[virtual potential temperature]], [[liquid water potential temperature|liquid water potential temperature]], [[equivalent potential temperature]], [[wet-bulb potential temperature]].</div><br/> </div> | ||
#<div class="definition"><div class="short_definition">In [[oceanography]], the [[temperature]] that a water sample would attain if raised [[adiabatically]] to the sea surface.</div><br/> <div class="paragraph">For the deepest points of the ocean, which are just over 10 000 m, the [[adiabatic cooling]] would be less than 1.5& | #<div class="definition"><div class="short_definition">In [[oceanography]], the [[temperature]] that a water sample would attain if raised [[adiabatic process|adiabatically]] to the sea surface.</div><br/> <div class="paragraph">For the deepest points of the ocean, which are just over 10 000 m, the [[adiabatic cooling]] would be less than 1.5°C.</div><br/> </div> | ||
</div> | </div> | ||
Latest revision as of 16:38, 25 April 2012
potential temperature
- The temperature that an unsaturated parcel of dry air would have if brought adiabatically and reversibly from its initial state to a standard pressure, p0, typically 100 kPa.
Its mathematical expression iswhere θ is the potential temperature, T is temperature, and κ is the Poisson constant. This exponent is often assumed to be 2/7, the ratio of the gas constant to the specific heat capacity at constant pressure for an ideal diatomic gas.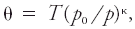
See virtual potential temperature, liquid water potential temperature, equivalent potential temperature, wet-bulb potential temperature.
- In oceanography, the temperature that a water sample would attain if raised adiabatically to the sea surface.
For the deepest points of the ocean, which are just over 10 000 m, the adiabatic cooling would be less than 1.5°C.
