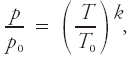Polytropic process: Difference between revisions
From Glossary of Meteorology
imported>Perlwikibot (Created page with " {{TermHeader}} {{TermSearch}} <div class="termentry"> <div class="term"> == polytropic process == </div> <div class="definition"><div class="short_definition">A thermo...") |
imported>Perlwikibot No edit summary |
||
| Line 9: | Line 9: | ||
</div> | </div> | ||
<div class="definition"><div class="short_definition">A thermodynamic process in which changes of [[pressure]] ''p'' and [[density]] ρ are related according to the formula <div class="display-formula"><blockquote>[[File:ams2001glos-Pe29.gif|link=|center|ams2001glos-Pe29]]</blockquote></div> where λ is a constant and subscript zeros denote initial values of the [[variables]].</div><br/> <div class="paragraph">Therefore pressure and [[temperature]] are similarly related: <div class="display-formula"><blockquote>[[File:ams2001glos-Pe30.gif|link=|center|ams2001glos-Pe30]]</blockquote></div> where ''k'' is the [[coefficient of polytropy]]. For [[isobaric]] processes, ''k'' = 0; for [[isosteric]] processes, ''k'' = 1; for [[adiabatic processes]] ''k'' = ''c''<sub>''p''</sub>/''R'', where ''c''<sub>''p''</sub> is the [[specific heat]] at constant pressure and ''R'' is the [[gas constant]]; sometimes applied to circumstances when adiabatic heating or cooling combine with slow ascent or descent to produce a particular [[lapse rate]]. In meteorology this formula is applied to individual [[air parcels]] and should be distinguished from that for a [[polytropic atmosphere]], which describes a distribution of pressure and temperature in space. <br/>''See also'' [[equation of piezotropy]].</div><br/> </div> | <div class="definition"><div class="short_definition">A thermodynamic process in which changes of [[pressure]] ''p'' and [[density]] ρ are related according to the formula <div class="display-formula"><blockquote>[[File:ams2001glos-Pe29.gif|link=|center|ams2001glos-Pe29]]</blockquote></div> where λ is a constant and subscript zeros denote initial values of the [[variables]].</div><br/> <div class="paragraph">Therefore pressure and [[temperature]] are similarly related: <div class="display-formula"><blockquote>[[File:ams2001glos-Pe30.gif|link=|center|ams2001glos-Pe30]]</blockquote></div> where ''k'' is the [[coefficient of polytropy]]. For [[isobaric]] processes, ''k'' = 0; for [[isosteric]] processes, ''k'' = 1; for [[adiabatic processes]] ''k'' = ''c''<sub>''p''</sub>/''R'', where ''c''<sub>''p''</sub> is the [[specific heat]] at constant pressure and ''R'' is the [[gas constant]]; sometimes applied to circumstances when adiabatic heating or cooling combine with slow ascent or descent to produce a particular [[lapse rate]]. In meteorology this formula is applied to individual [[air parcels]] and should be distinguished from that for a [[polytropic atmosphere]], which describes a distribution of pressure and temperature in space. <br/>''See also'' [[equation of piezotropy|equation of piezotropy]].</div><br/> </div> | ||
</div> | </div> | ||
Latest revision as of 17:38, 25 April 2012
polytropic process
A thermodynamic process in which changes of pressure p and density ρ are related according to the formula where λ is a constant and subscript zeros denote initial values of the variables.
Therefore pressure and temperature are similarly related: where k is the coefficient of polytropy. For isobaric processes, k = 0; for isosteric processes, k = 1; for adiabatic processes k = cp/R, where cp is the specific heat at constant pressure and R is the gas constant; sometimes applied to circumstances when adiabatic heating or cooling combine with slow ascent or descent to produce a particular lapse rate. In meteorology this formula is applied to individual air parcels and should be distinguished from that for a polytropic atmosphere, which describes a distribution of pressure and temperature in space.
See also equation of piezotropy.
See also equation of piezotropy.