Ph
From Glossary of Meteorology
pH[edit | edit source]
In aqueous solution, the logarithm (base 10) of the inverse of the concentration of hydrogen ions expressed in moles per liter; a measure of solution acidity. Thus, 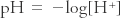 .
.
The pH of neutral water is 7, since it contains 10-7 moles hydrogen ions and 10-7 moles hydroxyl ions per liter. Lower pH values correspond to more acidic solution. The pH of natural waters in the atmosphere is 5.5 or less, due to the dissolution of acidic gases such as carbon dioxide and sulfur dioxide. In polluted continental areas the pH can drop to as low as 3.0, a condition known as acid rain, due to the production of sulfuric and nitric acids from anthropogenic activity.
