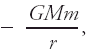Potential energy
From Glossary of Meteorology
potential energy
The energy a system has by virtue of its position; the negative of the work done in taking a system from a reference configuration, where the potential energy is assigned the value zero, to a given configuration, with no change in kinetic energy of the system.
An example of potential energy is the gravitational potential energy of a point mass m at a distance r from the center of a spherically symmetric body with mass M (e.g., a planet): where G is the universal gravitational constant and the reference potential energy is taken as zero at infinity. At distances z above the surface of the body that are small compared with its radius, the potential energy is approximately where g is the acceleration due to gravity at the surface and the zero of potential energy is taken at the surface (z = 0). Molecular potential energies, arising from short-range forces much stronger than gravitation, are involved in all chemical reactions, are responsible for the cohesiveness of liquids and solids, and influence a host of processes such as evaporation and condensation.